- Enforcement Directorate conducting searches at seven premises in Chennai tied to Sresan Pharmaceuticals.
- Money laundering case filed; owner Ranganathan Govindan in 10-day police custody.
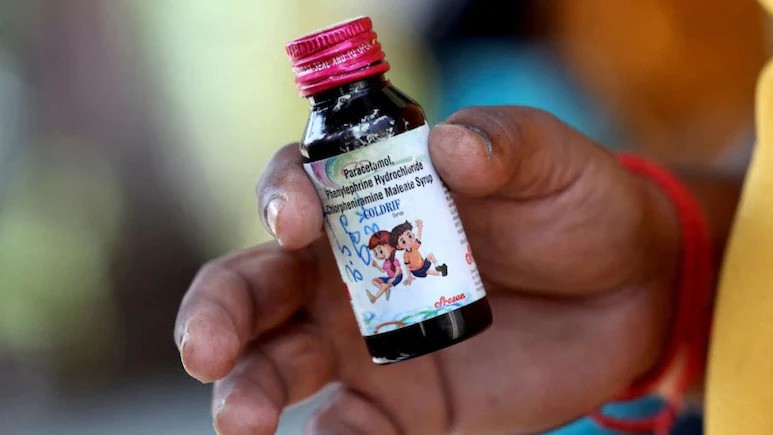
- ‘Coldrif’ syrup allegedly caused at least 20 children’s deaths in MP and Rajasthan; dangerously adulterated with diethylene glycol (DEG).
GG News Bureau
Chennai, 13th Oct: The Enforcement Directorate (ED) is conducting raids at seven locations in Chennai connected to Sresan Pharmaceuticals, the manufacturer of the poisonous ‘Coldrif’ cough syrup, which has been linked to at least 20 children’s deaths in Madhya Pradesh and Rajasthan. Authorities confirmed that the probe extends to the residences of senior officials in Tamil Nadu’s drug control office.
A money laundering case has been registered against the company. Its owner, Ranganathan Govindan, was arrested last week and placed in 10-day police custody in connection with the case. Investigations revealed that the syrup was adulterated with diethylene glycol (DEG), a highly toxic substance. The company’s Tamil Nadu plant reportedly had over 350 violations, including 38 serious breaches of national drug safety norms.
Sresan Pharmaceuticals, licensed in 2011 by the Tamil Nadu Food and Drug Administration (TNFDA) and based in Kanchipuram, operated for over a decade despite repeated violations. Its license was renewed in 2016, and the Central Drugs Standard Control Organisation (CDSCO) was not involved in the process. Following the deaths, several states, including Madhya Pradesh, Rajasthan, Tamil Nadu, and Delhi, have banned the syrup, while the Tamil Nadu government ordered closure of the firm and is considering permanent revocation of its license.
Amid nationwide outrage, the World Health Organisation (WHO) expressed concern over India’s regulatory gaps in screening for DEG and ethylene glycol in domestically sold medicines. The WHO highlighted the risk of contaminated products being exported, and urged authorities to identify and remove affected pharmaceutical materials. WHO has also reached out to the CDSCO for clarification on the matter.
The deadly incident has sparked calls for stricter oversight of pharmaceutical manufacturing and renewed scrutiny of drug regulatory mechanisms across India.